FDA Proposes New Sterilization Requirements For Medical Devices

The industry is moving away from ethylene oxide (EtO) sterilization, citing environmental impacts.
On January 8, 2024, the FDA (Food and Drug Administration) issued a press release stating that it revised its final guidance on submissions for devices labeled as sterile to permit the use of VHP (Vaporized Hydrogen Peroxide also known as VPHP) a Category A sterilization method. This should pave the path for companies to employ VHP where, in the past, ethylene oxide (EtO) sterilization was the method of choice.
Transitioning to a new sterilization method is a process that takes significant lead time. Let us guide you through the long process to help you meet critical deadlines that will be required by FDA.
Where Did This Decision Stem From?
This decision comes on the heels of public concerns about environmental hazards resulting from ethylene oxide, including a $408MM settlement in 2023 with medical device sterilizers in Illinois, with other cases pending.
Prior to this industry move, VHP was known as a sanitizer, but was not accepted by the FDA as a sterilization method, where sterilization carries the requirement of a 10-6 reduction in microbial growth, with sanitization having reduced requirements for microbial removal.
EPA Lowered EtO Emission Limits
As a partial mitigation to the health problems linked to EtO use, including cancer risks, the EPA (Environmental Protection Agency) lowered its EtO emissions limits in April of 2023, effective October 2024 to provide time for industry to adjust.
Databases Updated To Support Alternate Sterilization Methods
Inter-agency co-operative efforts then updated its Recognized Standards database to include standards and information supporting sterilization though low-temperature vaporized hydrogen peroxide as an alternative. Previously, the FDA did not recognize a standard method for VHP sterilization, resulting in industry shying from its use.
Now that the FDA recognizes this sterilization method, it is incumbent on industry to ensure that this method is properly employed, and more importantly, properly validated.
When VHP Should + Shouldn’t Be Used
VHP is inappropriate at times, and it is not foreseeable that EtO sterilization will be abandoned altogether in the near future. The table below outlines the advantages and limitations of each sterilization method, including VHP.
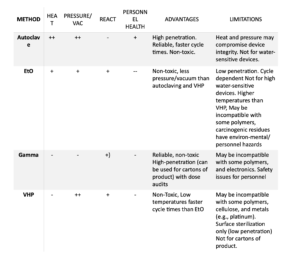
Proper use of VHP therefore requires verification that the method is compatible with the product. Similar to autoclaving, VHP cycles and loading patterns must be developed specific to the product, as multiple vacuum/injection cycles are necessary to assure full penetration of the hydrogen peroxide inside the material enclosing the product.
Understanding Hydrogen Peroxide Cycles
Unlike EtO or autoclaving, hydrogen peroxide requires several cycles to fully penetrate. Despite the multiple cycles, dwell times are reduced, resulting in a VHP cycle that is shorter than a similar EtO cycle.
Tyvek® Usage
Tyvek® or similar material is recommended for covering the device because of VHP’s incompatibility with cellulose and its difficulty in penetration. Furthermore, VHP must only be used for a device in its primary covering and cannot be used if the device is enclosed in outer packaging materials.
Consider The Risk Of Hydrogen Peroxide
Another limitation to consider is that residual hydrogen peroxide may be a risk for medical device compatibility and patient needs. The firm’s risk management program must consider these risks and validation testing may require establishment of residue limits and enhanced testing during validation and routine testing at product release
VHP is unique from other sterilization methods, in that hydrogen peroxide exists in equilibrium with water, and, as a free-radical, naturally degrades into water and oxygen on its own.
During cycle development, vacuum cycles must be tightly controlled to prevent humidity buildup and water condensation within the chamber, which has the potential to damage some medical devices. It is recommended that if a medical device is highly-sensitive to the presence of liquid water, avoid VHP.
Occluded Surfaces + Passive Diffusion Areas
Items to consider are occluded surfaces and passive diffusion areas. An occluded surface is an area or location which is completely obstructed from VHP exposure. An occluded surface will not be decontaminated as VHP has poor penetrating abilities.
Passive diffusion is the net movement of VHP-laden air from an area of high concentration to an area with lower concentration. Examples areas that may rely on passive diffusion of VHP would be Pipe Dead-Legs or within a container.
Validation
Once cycles are properly developed, validation is required to ensure that the process is repeatable. Similar to autoclave validation, it is recommended to perform at least some testing under a shortened cycle, and to specify the load pattern(s) for the product inside the chamber.
Biological indicators are like those used for autoclaving (Geobacillus stearothermophilus), but are developed for hydrogen peroxide penetration. Chemical indicators are strips coated with indicator ink that demonstrate a gradual color change upon exposure to hydrogen peroxide gas.
These indicators are used to visually assess the gas distribution through various locations in the target material. Sensors have been developed to measure conditions within the VHP chamber, to compare equipment settings to measurements within theoretical worst-case locations within the chamber to assure optimal sterilization performance.
How Will You Validate Your VPH Process?
We hope that this guide to VHP validation is helpful to you. The sources below provide more technical background if you would like to learn more.
To aid with this necessary transition, Compliance Team can assist with critical method verification and VPH process validation that will be mandated by FDA. Get in touch with us here.
Sources |
Parenteral Drug Association. “PDA Technical Report No. 34: Design and Validation of Isolator Systems for the Manufacturing and Testing of Health Care Products.” September 2001. |
Google search on January 17, 2024 “Biological Indicators for VHP” |
Reuter, Elise. EPA to Limit Ethylene Oxide Emissions from Medical Device Sterilizers.: April 11, 2023. MedTechDive. Accessed January 17, 2023 at EPA to limit ethylene oxide emissions from medical device sterilizers | MedTech Dive |
Taylor, Nick Paul. “Sterigenics’ $408M Ethylene Oxide Settlement Removes ‘Most Problematic’ Lawsuits: Analysts.” September 8, 2023. MedTechDive. Accessed January 17, 2023 at Sterigenics’ $408M ethylene oxide settlement removes ‘most problematic’ lawsuits: analysts | MedTech Dive |
PDA Journal of Pharmaceutical Science and Technology Points to Consider for Aseptic Processing. March/April 2003 |
PIC/S Secretariat, ed., “Guide to Good Manufacturing Practice for Medicinal Products.” Pharmaceutical Inspection Convention, PE 009-17 (Rev 2.) August 25, 2023. |
PIC/S Secretariat, ed., “Recommendation: Isolators Used for Aseptic Processing and Sterility Testing.” Pharmaceutical Inspection Convention, PI 014-3 September 25, 2007. |
Steris Applied Sterilization Technologies. “TechTalk: Fundamentals of Vaporized Hydrogen Peroxide” November 4, 2020, Accessed on January 17, 2024, at https://www.youtube.com/watch?v=1LZTdmzv69U&t=103s |
Technical Committee ISO/TC 198, Sterilization of health care products, “ISO 11138-1:2017(en) Sterilization of Health Care Products—Biological Indicators—Part 1: General Requirements.” 3rd Edition. March 2017. |
Technical Committee ISO/TC 198, Sterilization of health care products, “ISO 11140-1:2014(en) Sterilization of Health Care Products—Chemical Indicators—Part 1: General Requirements.” 3rd Edition November 2014. |
U.S. Food and Drug Administration. “CDRH Announces New Standards Recognition to Support Innovation in Medical Device Sterilization” July 24, 2023. Accessed January 17, 2023 at FDA Facilitates Broader Adoption of Vaporized Hydrogen Peroxide for Medical Device Sterilization | FDA |
U.S. Food and Drug Administration. “FDA Facilitates Broader Adoption of Vaporized Hydrogen Peroxide for Medical Device Sterilization—Agency Continues to Encourage Ethylene Oxide Sterilization Alternatives” January 8, 2024. Accessed January 17, 2023 at FDA Facilitates Broader Adoption of Vaporized Hydrogen Peroxide for Medical Device Sterilization | FDA |
U.S. Food and Drug Administration. “Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice” September 2004. Accessed January 18, 2024 at Guidance for Industry (fda.gov) |
Vaisala. H2O2 Conversions: Formulas and Methods for Calculating Vaporized Hydrogen Peroxide Parameters. 2022 Downloaded from Vaisala.com on January 17, 2024. |
Vaisala. Vaporized Hydrogen Peroxide, Humidity and Temperature Measurement HPP270 Series. Accessed on January 17, 2024 at Vaporized Hydrogen Peroxide, Humidity and Temperature Measurement HPP270 Series | Vaisala |